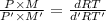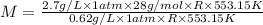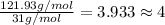Chemistry, 18.09.2019 02:00 davisparker5269
At its boiling point (280°c) and at atmospheric pressure, phosphorus gas has a density of 2.7 g l–1 . under the same conditions, nitrogen gas has a density of 0.62 g l–1 . how many atoms of phosphorus are there in one phosphorus molecule under these conditions?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
At its boiling point (280°c) and at atmospheric pressure, phosphorus gas has a density of 2.7 g l–1...
Questions




Biology, 06.07.2019 21:00

Arts, 06.07.2019 21:00


Mathematics, 06.07.2019 21:00



Geography, 06.07.2019 21:00



Mathematics, 06.07.2019 21:00

Mathematics, 06.07.2019 21:00

English, 06.07.2019 21:00

Geography, 06.07.2019 21:00

English, 06.07.2019 21:00


Biology, 06.07.2019 21:00

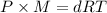 ...(1)
...(1)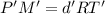
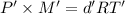 ...(2)
...(2)