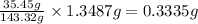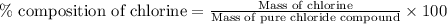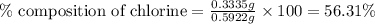Chemistry, 18.09.2019 03:30 shortcake8047
A0.5922 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride ion is precipitated as agcl by the addition of an excess of silver nitrate. the mass of the resulting agcl is found to be 1.3487 g. what is the mass percentage of chlorine in the original compound?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
A0.5922 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride...
Questions



Biology, 30.05.2020 02:58

Mathematics, 30.05.2020 02:58




Health, 30.05.2020 02:58


Mathematics, 30.05.2020 02:58

Spanish, 30.05.2020 02:58



Mathematics, 30.05.2020 02:58

Mathematics, 30.05.2020 02:58

Mathematics, 30.05.2020 02:58

Chemistry, 30.05.2020 02:58

Mathematics, 30.05.2020 02:58

Mathematics, 30.05.2020 02:58

Mathematics, 30.05.2020 02:58