Chemistry, 18.09.2019 03:30 Ruthsybel9754
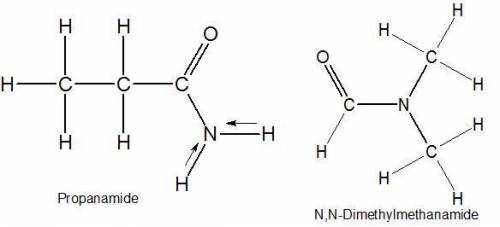
Select the single best answer. why is the boiling point of propanamide, ch3ch2conh2, considerably higher than the boiling point of n, n−dimethylformamide, hcon(ch3)2 (213°c vs. 153°c), even though both compounds are isomeric amides? ch3ch2conh2 has weaker intermolecular forces than hcon(ch3)2 hydrogen bonding is present in ch3ch2conh2 but not in hcon(ch3)2 ch3ch2conh2 is more branched than hcon(ch3)2 ch3ch2conh2 has two methyl groups

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
Select the single best answer. why is the boiling point of propanamide, ch3ch2conh2, considerably hi...
Questions



Mathematics, 18.12.2020 23:50

Mathematics, 18.12.2020 23:50


Mathematics, 18.12.2020 23:50





History, 18.12.2020 23:50

Geography, 18.12.2020 23:50


English, 18.12.2020 23:50

Biology, 18.12.2020 23:50



World Languages, 18.12.2020 23:50

Mathematics, 18.12.2020 23:50