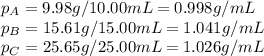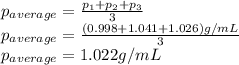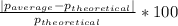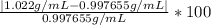Chemistry, 18.09.2019 04:10 christianskyy7074
Density measurements were conducted on a 22.5oc sample of water which had a theoretical density of 0.997655 g/ml. a volume of 10.00 ml of the water had a mass of 9.98 g. a volume of 15.00 ml of the water had a mass of 15.61 g. a volume of 25.00 ml of the water had a mass of 25.65 g. (1) show the calculation of the density of each volume. (2) show the calculation of the average density. (3) show the calculation of the percent error based on the theoretical density of 0.997655 g/ml.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
Density measurements were conducted on a 22.5oc sample of water which had a theoretical density of 0...
Questions

Health, 31.03.2021 18:10

Mathematics, 31.03.2021 18:10







Biology, 31.03.2021 18:10

Mathematics, 31.03.2021 18:10





Mathematics, 31.03.2021 18:10




Arts, 31.03.2021 18:10

Biology, 31.03.2021 18:10