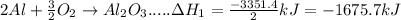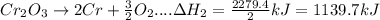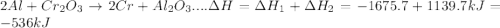In the following overall chemical reaction, aluminum displaces chromium from chromium(iii) oxide and forms aluminum oxide.
2 al(s) + cr2o3(s) → 2 cr(s) + al2o3(s); δh = ?
find the change in enthalpy for this reaction, using hess' law and the enthalpy changes for the reactions given below.
(1a) 4 al(s) + 3 o2(g) → 2 al2o3(s); δh = −3351.4 kj
(2a) 4 cr(s) + 3 o2(g) → 2 cr2o3(s); δh = −2279.4 kj

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
In the following overall chemical reaction, aluminum displaces chromium from chromium(iii) oxide and...
Questions


Mathematics, 03.11.2021 08:00

Mathematics, 03.11.2021 08:00

Mathematics, 03.11.2021 08:10


World Languages, 03.11.2021 08:10

Mathematics, 03.11.2021 08:10

History, 03.11.2021 08:10


Mathematics, 03.11.2021 08:20

Chemistry, 03.11.2021 14:00




Mathematics, 03.11.2021 14:00


Mathematics, 03.11.2021 14:00

Mathematics, 03.11.2021 14:00

English, 03.11.2021 14:00

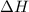 is an additive property. hence value of
is an additive property. hence value of