Chemistry, 18.09.2019 05:20 stephaniero6
Asample of liquefied natural, lng, from alaska has the following molar composition: 3.9% c2h6, 1.1% c3h8, 1.1% co2, and the rest ch4. determine (a) the average molecular weight of the lng mixture. (b) the weight fraction of ch4 in the mixture. (c) the lng is heated to 422 k and 148 kpa, and vaporizes completely. estimate the density of the gas mixture under these conditions.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
Asample of liquefied natural, lng, from alaska has the following molar composition: 3.9% c2h6, 1.1%...
Questions

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

History, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00



Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

English, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

English, 25.03.2021 01:00


English, 25.03.2021 01:00



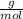
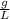
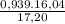 ×100 = 87,57%
×100 = 87,57%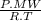 = δ
= δ