
Chemistry, 18.09.2019 05:20 capybaracaptin2895
A36.0 mg sample of an organic compound (molar mass = 84.0 g/mol) is dissolved in 10.0 ml of water. this aqueous solution is extracted with 5.0 ml of hexane. separation and analysis of the aqueous phase shows that it now contains 12.0 mg of the organic compound. calculate the partition coefficient for the compound between hexane and water (kphexane/water). your answer should reflect the proper number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3


You know the right answer?
A36.0 mg sample of an organic compound (molar mass = 84.0 g/mol) is dissolved in 10.0 ml of water. t...
Questions

History, 11.04.2020 01:13

Mathematics, 11.04.2020 01:13

Mathematics, 11.04.2020 01:13



Chemistry, 11.04.2020 01:13


History, 11.04.2020 01:13


Mathematics, 11.04.2020 01:13

Mathematics, 11.04.2020 01:13

Mathematics, 11.04.2020 01:13


Physics, 11.04.2020 01:13


Biology, 11.04.2020 01:13

History, 11.04.2020 01:13



Mathematics, 11.04.2020 01:14

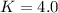
![[solute]_{water}=\frac{12mg*\frac{1mmol}{84.0mg}}{10.0mL}*\frac{1000mL}{1L}=14.3mM](/tpl/images/0238/7804/ef1af.png)
![[solute]_{hexane}=\frac{(36.0-12.0)mg*\frac{1mmol}{84.0mg}}{5mL}*\frac{1000mL}{1L}=57mM](/tpl/images/0238/7804/17da7.png)
![K=\frac{[solute]_{hexane}}{[solute]_{water}} = \frac{57mM}{14.3mM} \\K=4.0](/tpl/images/0238/7804/d9577.png)


