
Chemistry, 18.09.2019 05:30 wowihavefun
A1.0 l sample of an aqueous solution contains 0.10 mol of nacl and 0.10 mol of cacl2. what is the minimum number of moles of agno3 that must be added to the solution in order to precipitate all of the cl- as agcl(s) ? (assume that agcl is insoluble.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
A1.0 l sample of an aqueous solution contains 0.10 mol of nacl and 0.10 mol of cacl2. what is the mi...
Questions


Mathematics, 12.02.2021 02:10





Social Studies, 12.02.2021 02:10


Mathematics, 12.02.2021 02:10


Mathematics, 12.02.2021 02:10


Mathematics, 12.02.2021 02:10






Computers and Technology, 12.02.2021 02:10

Mathematics, 12.02.2021 02:10

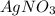 to precipitate all of the
to precipitate all of the 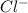 ions.
ions.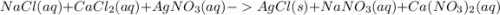
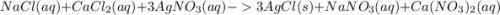
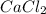 , we need 3 moles of
, we need 3 moles of 

