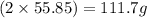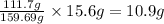Chemistry, 18.09.2019 17:10 michellemonroe012305
A29.7 g sample of iron ore is treated as follows. the iron in the sample is all converted by a series of chemical reactions to fe₂o₃. the mass of fe₂o₃ is measured to be 15.6 g. what was the mass of iron in the sample of ore? answer in units of g.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
A29.7 g sample of iron ore is treated as follows. the iron in the sample is all converted by a serie...
Questions

Mathematics, 23.07.2020 21:01





Computers and Technology, 23.07.2020 21:01

Mathematics, 23.07.2020 21:01






Social Studies, 23.07.2020 21:01