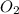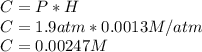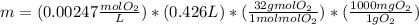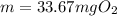Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
What mass of oxygen gas, in mg, is dissolved in 4.26×102 ml of water when the partial pressure of ox...
Questions


Mathematics, 26.08.2020 01:01




Mathematics, 26.08.2020 01:01

Physics, 26.08.2020 01:01

Mathematics, 26.08.2020 01:01


Advanced Placement (AP), 26.08.2020 01:01



Mathematics, 26.08.2020 01:01




Chemistry, 26.08.2020 01:01

Mathematics, 26.08.2020 01:01