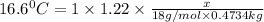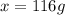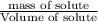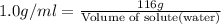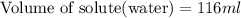Chemistry, 18.09.2019 22:00 shoafmckenzie5263
What volume of water (solute) (d = 1.00 g/ml) should be added to 600. ml of ethanol (solvent, c2h5oh) in order to have a solution that boils at 95.0°c? [for ethanol, kb = 1.22 °c/m, density = 0.789 g/ ml, boiling point = 78.4°c]

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 11:30
If this sedimentary rock layer is truly the oldest one of marine origin, what do you think that tells usabout the formation of earth's oceans?
Answers: 2

Chemistry, 23.06.2019 13:00
How many grams of oxygen gas will react completely with a block of calcium metal that is 3.0 cm by 3.5 cm by 4.2 cm, if the density of calcium is 1.55 g/ml? show all steps of your calculation as well as the final answer.
Answers: 3

Chemistry, 23.06.2019 14:00
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1
You know the right answer?
What volume of water (solute) (d = 1.00 g/ml) should be added to 600. ml of ethanol (solvent, c2h5oh...
Questions

Mathematics, 27.06.2019 08:30


Biology, 27.06.2019 08:30

Mathematics, 27.06.2019 08:30



History, 27.06.2019 08:30






History, 27.06.2019 08:30





Health, 27.06.2019 08:30


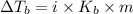
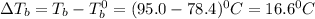 = elevation in boiling point
= elevation in boiling point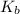 =boiling point constant =
=boiling point constant = 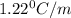
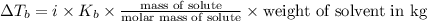
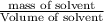
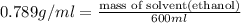
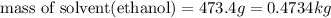 (1kg=1000g)
(1kg=1000g)