
Chemistry, 18.09.2019 22:00 camiloriveraveoxbgd6
2mno₄ - (aq) + 5 c₂o₄²⁻ (aq) + 16 h⁺ (aq) → 2 mn²⁺ (aq) + 10 co₂ (g) + 8 h₂o (l)permanganate and oxalate ions react in an acidified solution according to the balanced equation above. how many moles of co₂(g) are produced when 20. ml of acidified 0.20 m kmno₄ solution is added to 50. ml of 0.10 m na₂c₂o₄ solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

You know the right answer?
2mno₄ - (aq) + 5 c₂o₄²⁻ (aq) + 16 h⁺ (aq) → 2 mn²⁺ (aq) + 10 co₂ (g) + 8 h₂o (l)permanganate and oxa...
Questions


History, 05.10.2021 14:00

Social Studies, 05.10.2021 14:00

Mathematics, 05.10.2021 14:00

Arts, 05.10.2021 14:00


English, 05.10.2021 14:00

Mathematics, 05.10.2021 14:00





Social Studies, 05.10.2021 14:00

SAT, 05.10.2021 14:00

Chemistry, 05.10.2021 14:00


Social Studies, 05.10.2021 14:00

SAT, 05.10.2021 14:00


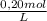 = 4,0×10⁻³ KMnO₄ moles
= 4,0×10⁻³ KMnO₄ moles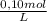 = 5,0×10⁻³ Na₂C₂O₄ moles
= 5,0×10⁻³ Na₂C₂O₄ moles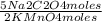 =
=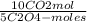 =
= 

