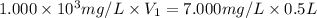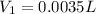Chemistry, 19.09.2019 01:00 student0724
If a chemist wishes to dilute a 1.000 × 10^3 mg/l stock solution to prepare 5.000 × 10^2 ml of a working standard that has a concentration of 7.000 mg/l, what volume of the 1.000 × 10^3 mg/l standard solution is needed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
If a chemist wishes to dilute a 1.000 × 10^3 mg/l stock solution to prepare 5.000 × 10^2 ml of a wor...
Questions


History, 19.09.2019 11:10


Chemistry, 19.09.2019 11:10

History, 19.09.2019 11:10

Mathematics, 19.09.2019 11:10



Computers and Technology, 19.09.2019 11:10

Mathematics, 19.09.2019 11:10

Mathematics, 19.09.2019 11:10


Mathematics, 19.09.2019 11:10

Biology, 19.09.2019 11:10

Mathematics, 19.09.2019 11:10

Mathematics, 19.09.2019 11:10


English, 19.09.2019 11:10


Chemistry, 19.09.2019 11:20

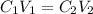
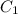 = molarity of stock solution =
= molarity of stock solution = 
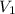 = volume of stock solution = ?
= volume of stock solution = ?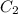 = molarity of diluted solution =
= molarity of diluted solution = 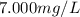
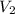 = volume of diluted solution =
= volume of diluted solution = 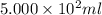 = 0.5 L (1L=1000 ml)
= 0.5 L (1L=1000 ml)