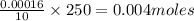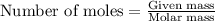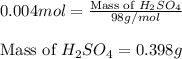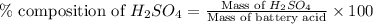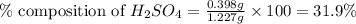Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.227 g . this 1.00-ml sample is diluted to 250.0 ml, and 10.00 ml of this diluted acid requires 35.05 ml of 4.462×10−3 m ba(oh)2 for its titration. what is the mass percent of h2so4 in the battery acid?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2


You know the right answer?
Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.2...
Questions

Social Studies, 20.07.2019 15:30









English, 20.07.2019 15:30





Computers and Technology, 20.07.2019 15:30


History, 20.07.2019 15:30

Computers and Technology, 20.07.2019 15:30

Mathematics, 20.07.2019 15:30

Physics, 20.07.2019 15:30

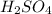 in the battery acid is 31.9 %
in the battery acid is 31.9 %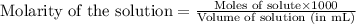
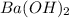 solution =
solution = 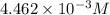
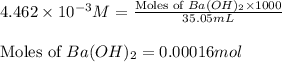
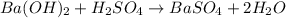
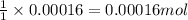 of sulfuric acid.
of sulfuric acid.