
Asolution contains 0.1 (10-7) moles of hydroxyl ions (oh-) per liter. which of the following best describes this solution? a solution contains 0.1 (10-7) moles of hydroxyl ions (oh-) per liter. which of the following best describes this solution? basic: h+ donor basic: h+ acceptor acidic: h+ acceptor neutral acidic: h+ donor

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
Asolution contains 0.1 (10-7) moles of hydroxyl ions (oh-) per liter. which of the following best de...
Questions

Physics, 23.08.2020 02:01

Physics, 23.08.2020 02:01


English, 23.08.2020 02:01

Mathematics, 23.08.2020 02:01


History, 23.08.2020 02:01



Mathematics, 23.08.2020 02:01




Advanced Placement (AP), 23.08.2020 02:01

Chemistry, 23.08.2020 02:01

Mathematics, 23.08.2020 02:01


Mathematics, 23.08.2020 02:01


Health, 23.08.2020 02:01

![pH=-\log [H^+]](/tpl/images/0241/8360/37e81.png)
![pOH=-\log[OH^-]](/tpl/images/0241/8360/fe336.png)
![pOH=-\log[10^{-7}]](/tpl/images/0241/8360/492ed.png)
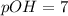
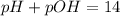
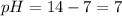
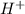 nor donates
nor donates 

