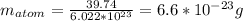Chemistry, 19.09.2019 03:30 itsgiovanna
The mass of a monoatomic gas can be computed from its specific heat at constant volume cv. (note that this is not cv.) take cv = 0.075 cal/g · c° for a gas and calculate (a) the mass of its atom and (b) the molar mass.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
The mass of a monoatomic gas can be computed from its specific heat at constant volume cv. (note tha...
Questions







English, 12.07.2019 17:20

World Languages, 12.07.2019 17:20

Spanish, 12.07.2019 17:20


History, 12.07.2019 17:20

Biology, 12.07.2019 17:20

History, 12.07.2019 17:20



English, 12.07.2019 17:20

Mathematics, 12.07.2019 17:20

History, 12.07.2019 17:20


Mathematics, 12.07.2019 17:20

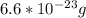
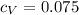 cal/g if the monoatomic gas behaves as ideal gas, we can know the molar specific heat at constant volume
cal/g if the monoatomic gas behaves as ideal gas, we can know the molar specific heat at constant volume 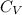 . For a monoatomical gas
. For a monoatomical gas 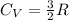 with R the universal gas constant. So
with R the universal gas constant. So 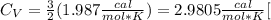 . We can calculate the heat with
. We can calculate the heat with 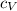 or
or 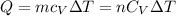 with m: mass and n: number of moles. So we can solve that equation for the molar mass (M=m/n) obtaining
with m: mass and n: number of moles. So we can solve that equation for the molar mass (M=m/n) obtaining 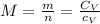 . We can answer the question b) M=2.9805/0.075=39.74 g/molWith the molar mass and the avogadro's number (
. We can answer the question b) M=2.9805/0.075=39.74 g/molWith the molar mass and the avogadro's number (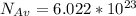
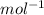 we can calculate the atom mass as follows
we can calculate the atom mass as follows 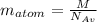 Answer for the question a) is
Answer for the question a) is