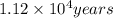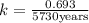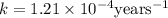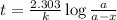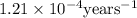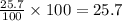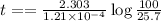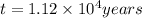Archaeologists can determine the age of artifacts made of wood or bone by measuring the amount of the radioactive isotope 14c present in the object. the amount of isotope decreases in a first-order process. if 25.7% of the original amount of 14c is present in a wooden tool at the time of analysis, what is the age of the tool? the half-life of 14c is 5,730 yr. give your answer in scientific notation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Archaeologists can determine the age of artifacts made of wood or bone by measuring the amount of th...
Questions

Mathematics, 15.12.2020 17:00



Advanced Placement (AP), 15.12.2020 17:00

Mathematics, 15.12.2020 17:00





Chemistry, 15.12.2020 17:00

Mathematics, 15.12.2020 17:00

Mathematics, 15.12.2020 17:00

English, 15.12.2020 17:00

Social Studies, 15.12.2020 17:00