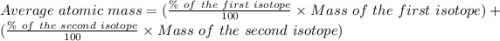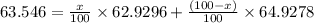Chemistry, 19.09.2019 16:30 xxaurorabluexx
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mass 64.9278 amu). if copper has an atomic mass of 63.546 amu, what is the percent abundance of each isotope? report your answer to 5 significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3


Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mas...
Questions



Chemistry, 14.01.2020 03:31

Social Studies, 14.01.2020 03:31


Computers and Technology, 14.01.2020 03:31



Social Studies, 14.01.2020 03:31

History, 14.01.2020 03:31




Computers and Technology, 14.01.2020 03:31