
Acompound of formula xcl3 reacts with aqueous agno3 to yield solid agcl according to the following equation: xcl3(aq)+3agno3(aq)→x(no3)3(aq)+3ag cl(s) when a solution containing 0.521 g of xcl3 was allowed to react with an excess of aqueous agno3, 1.68 g of solid agcl was formed. what is the identity of the atom x?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1


Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Acompound of formula xcl3 reacts with aqueous agno3 to yield solid agcl according to the following e...
Questions

Mathematics, 13.04.2021 20:50




Mathematics, 13.04.2021 20:50

English, 13.04.2021 20:50

Physics, 13.04.2021 20:50

Mathematics, 13.04.2021 20:50

Mathematics, 13.04.2021 20:50

History, 13.04.2021 20:50

Mathematics, 13.04.2021 20:50



Mathematics, 13.04.2021 20:50


English, 13.04.2021 20:50

Chemistry, 13.04.2021 20:50



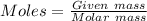
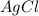 = 1.68 g
= 1.68 g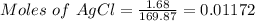
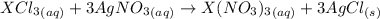
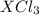 undergoes reaction.
undergoes reaction.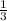 mole of
mole of 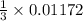 mole of
mole of 

