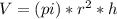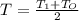An ideal gas in a cylindrical container of radius r and height h is kept at constant pressure p. the bottom of the container is maintained at temperature t0 while the top at temperature t1. assuming a linear temperature distribution along the cylinder, calculate the total mass of gas within the container.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

You know the right answer?
An ideal gas in a cylindrical container of radius r and height h is kept at constant pressure p. the...
Questions

Mathematics, 27.11.2021 23:40

Mathematics, 27.11.2021 23:40


English, 27.11.2021 23:40

Mathematics, 27.11.2021 23:40

Mathematics, 27.11.2021 23:40


Biology, 27.11.2021 23:40


History, 27.11.2021 23:40

Computers and Technology, 27.11.2021 23:40






Mathematics, 27.11.2021 23:40

Chemistry, 27.11.2021 23:40


Biology, 27.11.2021 23:40

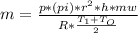
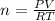
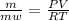 or
or  (equation 2)
(equation 2)