
Chemistry, 19.09.2019 20:30 lulabelles7750
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0 liter container was found to contain 1.60 moles of a, and 0.40 moles of b, and 0.40 moles of c, and 1.60 moles of d. calculate kc.(b) if 0.20 moles of b and 0.20 mole of c are added to this system, what will be the new equilibrium concentration of a be? (c) if the volume of the container in which the system is at equilibrium [part (a)] is suddenly halved, what will be the new equilibrium concentrations?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

Chemistry, 23.06.2019 13:30
Which of these statements describes the size of an atom? a. an atom is larger than a sheet of aluminum foil. b. an atom is small but can be seen with just our eyes. c. an atom is the size of a plastic building block. d. an atom is tiny and cannot be seen without magnification.
Answers: 2
You know the right answer?
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0...
Questions

Mathematics, 26.10.2020 21:10

Mathematics, 26.10.2020 21:10

Mathematics, 26.10.2020 21:20

Social Studies, 26.10.2020 21:20


Social Studies, 26.10.2020 21:20


Mathematics, 26.10.2020 21:20

Physics, 26.10.2020 21:20


Mathematics, 26.10.2020 21:20

Advanced Placement (AP), 26.10.2020 21:20

Computers and Technology, 26.10.2020 21:20

Mathematics, 26.10.2020 21:20





Biology, 26.10.2020 21:20

![\frac{[C]^2}{[A][B]}](/tpl/images/0244/0592/0c73c.png)
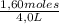 = 0,4 M
= 0,4 M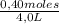 = 0,1 M
= 0,1 M![\frac{[0,1]^2}{[0,4][0,1]}](/tpl/images/0244/0592/6198c.png) = 0,25
= 0,25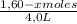
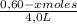
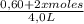
![\frac{[0,60+2x]^2}{[1,60-x][0,60-x]}](/tpl/images/0244/0592/06e92.png)
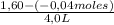 = 0,41 M
= 0,41 M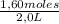 = 0,8 M
= 0,8 M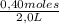 = 0,2 M
= 0,2 M

