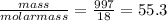Liquids and solids are left out of the equilibrium constant expression because their concentrations remain constant during reactions. what is the molarity concentration of liquid water at 25.0 latex: ^\circ ∘c given that its density is 0.997 g/ml at that temperature?
a.23.5 m
b.0.997 m
c.0.180 m
d.0.156 m
e.55.3 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Liquids and solids are left out of the equilibrium constant expression because their concentrations...
Questions




Computers and Technology, 14.10.2019 07:30



Mathematics, 14.10.2019 07:30




Mathematics, 14.10.2019 07:30


Social Studies, 14.10.2019 07:30




Physics, 14.10.2019 07:30



Health, 14.10.2019 07:30