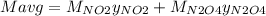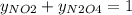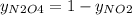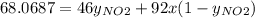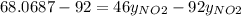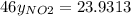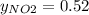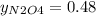Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
Nitrogen dioxide (no2) cannot be obtained in a pure form in the gas phase because it exists as a mix...
Questions




World Languages, 27.01.2020 09:31

Mathematics, 27.01.2020 09:31

Mathematics, 27.01.2020 09:31

Geography, 27.01.2020 09:31



Mathematics, 27.01.2020 09:31





Mathematics, 27.01.2020 09:31


Mathematics, 27.01.2020 09:31



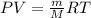
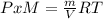
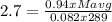
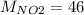 g/mol and
g/mol and 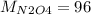 g/mol. Calling y the molar fraction:
g/mol. Calling y the molar fraction: