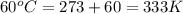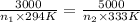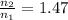Chemistry, 20.09.2019 03:10 arulanreddy3754
Ahot-air balloon is filled with air to a volume of 3000 m3 at 750 torr and 21°c. the air in the balloon is then heated to 60.°c, causing the balloon to expand to a volume of 5000. what is the ratio of the number of moles of air in the heated balloon to the original number of moles of air in the balloon? (hint: openings in the balloon allow air to flow in and out. thus the pressure in the balloon is always the same as that of the atmosphere.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

You know the right answer?
Ahot-air balloon is filled with air to a volume of 3000 m3 at 750 torr and 21°c. the air in the ball...
Questions


History, 02.01.2020 01:31

Mathematics, 02.01.2020 01:31

Social Studies, 02.01.2020 01:31

History, 02.01.2020 01:31




Mathematics, 02.01.2020 01:31

Mathematics, 02.01.2020 01:31




Social Studies, 02.01.2020 01:31


Computers and Technology, 02.01.2020 01:31




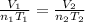
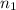 =original number of moles of air in the balloon = ?
=original number of moles of air in the balloon = ?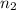 = number of moles of air in the heated balloon = ?
= number of moles of air in the heated balloon = ?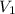 = initial volume of gas =
= initial volume of gas = 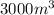
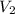 = final volume of gas =
= final volume of gas = 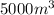
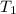 = initial temperature of gas =
= initial temperature of gas = 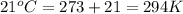
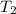 = final temperature of gas =
= final temperature of gas =