Chemistry, 20.09.2019 04:00 bryantpropst1395
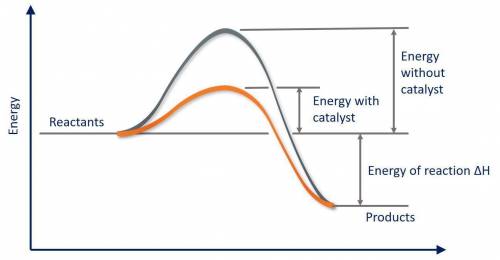
Which of the following statements about catalysts is false? a catalyst provides a different reaction pathway with a lower activation energy. a catalyst cannot affect the overall energy change for the reaction. a catalyst speeds up both the forward and reverse reactions. a catalyst is present before the reaction begins, and is also present in the same form after the reaction ends. a catalyst stabilizes the product of the reaction relative to the reactant.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1


Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
Which of the following statements about catalysts is false? a catalyst provides a different reactio...
Questions

Geography, 21.08.2019 05:30

Advanced Placement (AP), 21.08.2019 05:30

Mathematics, 21.08.2019 05:30



Mathematics, 21.08.2019 05:30


English, 21.08.2019 05:30

Mathematics, 21.08.2019 05:30




History, 21.08.2019 05:30

Social Studies, 21.08.2019 05:30


Mathematics, 21.08.2019 05:30

Biology, 21.08.2019 05:30

Mathematics, 21.08.2019 05:30

History, 21.08.2019 05:30

Social Studies, 21.08.2019 05:30