
Chemistry, 20.09.2019 17:30 student0724
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (molar mass = 18.01 g/mol) at 25°c. what is the vapor pressure of the solution if the vapor pressure of water at 25°c is 23.76 mm hg?
a) 20.5 mm hg
b) 22.1 mm hg
c) 22.9 mm hg
d) 24.7 mm hg

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

You know the right answer?
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (m...
Questions

Mathematics, 16.04.2021 20:00

Mathematics, 16.04.2021 20:00

Computers and Technology, 16.04.2021 20:00




Mathematics, 16.04.2021 20:00




Arts, 16.04.2021 20:00

Mathematics, 16.04.2021 20:00



Mathematics, 16.04.2021 20:00



Social Studies, 16.04.2021 20:00

History, 16.04.2021 20:00

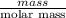
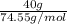
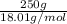
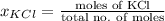
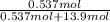
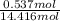
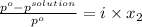
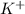 and
and 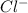 .
.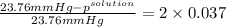
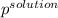 = 22.1 mm Hg
= 22.1 mm Hg

