
The elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 690 k is 1.27 × 101 m-1s-1. the reaction half-life at this temperature when [no2]0 = 0.45 m is s. the elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 690 k is 1.27 × 101 m-1s-1. the reaction half-life at this temperature when [no2]0 = 0.45 m is s. 0.17 18 5.7 0.054 0.079

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
The elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 6...
Questions







Computers and Technology, 04.09.2020 23:01

Mathematics, 04.09.2020 23:01


Mathematics, 04.09.2020 23:01




Mathematics, 04.09.2020 23:01



Mathematics, 04.09.2020 23:01


Computers and Technology, 04.09.2020 23:01

Mathematics, 04.09.2020 23:01

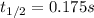
![\frac{1}{[NO_2]}=kt+ \frac{1}{[NO_2]_0}](/tpl/images/0248/0810/446e1.png)
![[NO_2]=\frac{[NO_2]_0}{2} \\](/tpl/images/0248/0810/70bce.png)
![t_{1/2}=\frac{1}{k[NO_2]_0} \\t_{1/2}=\frac{1}{(1.27x10^1\frac{L}{mol*s} )(0.45 \frac{mol}{L} )} \\\\t_{1/2}=0.175s](/tpl/images/0248/0810/f2ea3.png)


