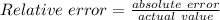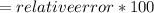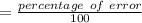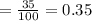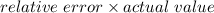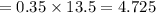Another student measured the volume of an unmarked water container, obtaining a 35.0% (6pts) given the actual volume of the container error at the end of the experiment. was 13.5l, what was the relative error, and the experimental measurement by the student? (assume re is +)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 12:30
What would happen to a weak base dissociation equilibrium if more products we added
Answers: 1

Chemistry, 23.06.2019 15:00
What is the volume in liters of 7500 g of helium atoms. assume stp conditions.
Answers: 1
You know the right answer?
Another student measured the volume of an unmarked water container, obtaining a 35.0% (6pts) given t...
Questions

Mathematics, 14.01.2021 02:10

World Languages, 14.01.2021 02:10

French, 14.01.2021 02:10


History, 14.01.2021 02:10

Mathematics, 14.01.2021 02:10

Computers and Technology, 14.01.2021 02:10

History, 14.01.2021 02:10





English, 14.01.2021 02:10

Health, 14.01.2021 02:10






Mathematics, 14.01.2021 02:10