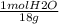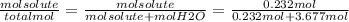Chemistry, 21.09.2019 21:30 floodlife4223
An aqueous solution is listed as being 33.8% solute by mass with a density of 1.15 g/ml, the molar mass of the solute is 145.6 g/mol and the molar mass of water is 18.0 g/mol. a) what is the molality of the solution? b) what is the mole fraction of the solute?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
An aqueous solution is listed as being 33.8% solute by mass with a density of 1.15 g/ml, the molar m...
Questions

Mathematics, 25.08.2019 01:10

Chemistry, 25.08.2019 01:10




Mathematics, 25.08.2019 01:10

Health, 25.08.2019 01:10

Mathematics, 25.08.2019 01:10


Mathematics, 25.08.2019 01:10


History, 25.08.2019 01:10



Mathematics, 25.08.2019 01:10




Physics, 25.08.2019 01:10

History, 25.08.2019 01:10

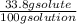 x
x 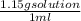 x
x 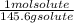 x
x 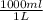
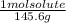 = 0.232 mol
= 0.232 mol