
Chemistry, 21.09.2019 23:10 ayoismeisalex
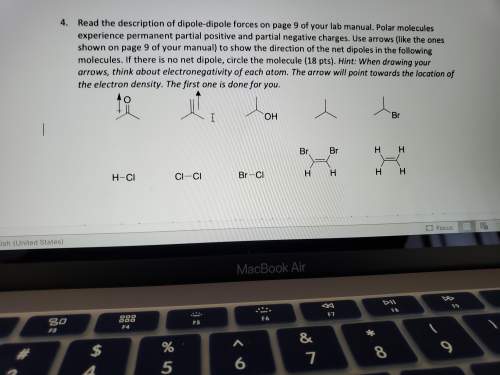
Read the description of dipole-dipole forces on page 9 of your lab manual. polar molecules experience permanent partial positive and partial negative charges. use arrows (like the ones shown on page 9 of your manual) to show the direction of the net dipoles in the following molecules. if there is no net dipole, circle the molecule (18 pts). hint: when drawing your arrows, think about electronegativity of each atom. the arrow will point towards the location of the electron density. the first one is done for you.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2
You know the right answer?
Read the description of dipole-dipole forces on page 9 of your lab manual. polar molecules experienc...
Questions

Mathematics, 11.01.2021 01:10


History, 11.01.2021 01:10


Mathematics, 11.01.2021 01:10



Mathematics, 11.01.2021 01:10

Mathematics, 11.01.2021 01:10


Biology, 11.01.2021 01:10



Mathematics, 11.01.2021 01:10


Mathematics, 11.01.2021 01:10

Mathematics, 11.01.2021 01:10



Mathematics, 11.01.2021 01:10



