

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3


Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
Calculate the enthalpy change for the reaction: caf2 + h2so4 → 2hf + caso4 given that enthalpy chan...
Questions



Mathematics, 04.03.2021 15:00

Mathematics, 04.03.2021 15:00

World Languages, 04.03.2021 15:00


Mathematics, 04.03.2021 15:00

Computers and Technology, 04.03.2021 15:00


Mathematics, 04.03.2021 15:00

Mathematics, 04.03.2021 15:00

Business, 04.03.2021 15:00

Mathematics, 04.03.2021 15:00


Geography, 04.03.2021 15:00



Mathematics, 04.03.2021 15:10

History, 04.03.2021 15:10

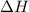
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0251/0819/db29b.png)
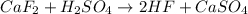
![\Delta H_{rxn}=[(2\times \Delta H_f_{(HF)})+(1\times \Delta H_f_{(CaSO_4)})]-[(1\times \Delta H_f_{(CaF_2)})+(1\times \Delta H_f_{(H_2SO_4)})]](/tpl/images/0251/0819/885d7.png)
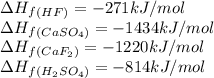
![\Delta H_{rxn}=[(2\times (-271))+(1\times (-1434))]-[(1\times (-1220))+(1\times (-814))]\\\\\Delta H_{rxn}=58kJ](/tpl/images/0251/0819/3f566.png)


