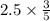Chemistry, 22.09.2019 04:30 sanociahnoel
The combustion of propane (c3h8) in the presence of excess oxygen yields co2 and h20: c3h8 (8) + 5o2 (g) - 3co2 (s) + 4h20 (8) when 2.5 mol of o2 are consumed in their reaction, a) 3.0 b) 7.5 c) 1.5 mol of co2 are produced. d) 4.2 e) 2.5

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
The combustion of propane (c3h8) in the presence of excess oxygen yields co2 and h20: c3h8 (8) + 5o...
Questions

English, 04.12.2019 02:31


Biology, 04.12.2019 02:31



Mathematics, 04.12.2019 02:31


Mathematics, 04.12.2019 02:31



Mathematics, 04.12.2019 02:31



History, 04.12.2019 02:31

Mathematics, 04.12.2019 02:31

Mathematics, 04.12.2019 02:31

Mathematics, 04.12.2019 02:31

Chemistry, 04.12.2019 02:31


Mathematics, 04.12.2019 02:31