
Chemistry, 23.09.2019 18:00 townykid96
There are three naturally occurring isotopes of the hypothetical element hilarium 45hi, 46hi, and 48hi. the percentages of these isotopes are the following: hilarium natural isotopes abundance 45hi 18.3% 46hi 34.5% 48hi 47.2% you can assume that the atomic masses are exactly equal to the mass number. calculate the weight of "naturally" occurring hilarium and report it as you would for any other naturally occurring element. answer in units of g/mol. your answer must be within ± 0.005%

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
There are three naturally occurring isotopes of the hypothetical element hilarium 45hi, 46hi, and 48...
Questions




Spanish, 06.05.2020 04:01

History, 06.05.2020 04:01

History, 06.05.2020 04:01

Physics, 06.05.2020 04:01

Mathematics, 06.05.2020 04:01

Mathematics, 06.05.2020 04:01


Geography, 06.05.2020 04:01

History, 06.05.2020 04:01

History, 06.05.2020 04:01



History, 06.05.2020 04:01

Mathematics, 06.05.2020 04:01



Mathematics, 06.05.2020 04:01

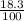 ) + (46 x
) + (46 x 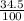 ) + (48 x
) + (48 x 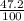 )
)

