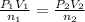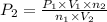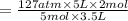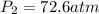Acetylene torches are used for welding. these torches use a mixture of acetylene gas, c2h2, and oxygen gas, o2 to produce the following combustion reaction: 2 c2h2 (g) + 5 o2 (g) → 4 co2 (g) + 2 h2o (g) imagine that you have a 5 l gas tank and a 3.5 l gas tank. you need to fill one tank with oxygen and the other with acetylene to use in conjunction with your welding torch. if you fill the larger tank with oxygen to a pressure of 127 atm , to what pressure should you fill the acetylene tank to ensure that you run out of each gas at the same time? assume ideal behavior for all gases.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2


Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
Acetylene torches are used for welding. these torches use a mixture of acetylene gas, c2h2, and oxyg...
Questions



English, 08.09.2021 17:40

Spanish, 08.09.2021 17:40

History, 08.09.2021 17:40



English, 08.09.2021 17:40


Chemistry, 08.09.2021 17:40


Physics, 08.09.2021 17:40





History, 08.09.2021 17:40

English, 08.09.2021 17:40

Mathematics, 08.09.2021 17:40

Social Studies, 08.09.2021 17:40

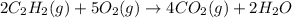
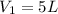
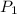 = 127 atm
= 127 atm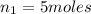
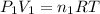
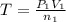 ..[1]
..[1]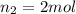
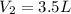
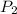 = ?
= ?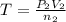 ..[2]
..[2]