
Chemistry, 24.09.2019 01:20 RickandMorty420710
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic oxygen, o2. the products and reactants are in a closed container and can all be treated as ideal gases. a. fill in the smallest possible integers that allows the stoichiometry of the reaction equation to be correct: __ kclo3 → kcl o2b. if there are n molecules of potassium chlorate in the initial state, how many product molecules are there

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic...
Questions

Mathematics, 27.10.2020 21:50


English, 27.10.2020 21:50


Mathematics, 27.10.2020 21:50



History, 27.10.2020 21:50

Biology, 27.10.2020 21:50

Arts, 27.10.2020 21:50

Health, 27.10.2020 21:50

Biology, 27.10.2020 21:50








Mathematics, 27.10.2020 21:50

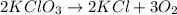
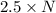 molecules of product.
molecules of product.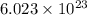 of particles.
of particles.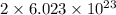 molecules of reactant give
molecules of reactant give 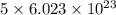 molecules of product
molecules of product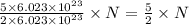 molecules of product.
molecules of product.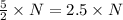 molecules of product are there.
molecules of product are there.

