
Chemistry, 24.09.2019 03:20 haydencheramie
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen molecules) into the engine. consider a car that is using 100% ethanol, c2h5oh, as fuel. if your engine intakes 4.73 l of air per minute at 1.00 atm and 25ºc, what is the maximum volume of ethanol (0.789 g/ml) that can be burned per minute? hint: you can ignore the "per minute" information because both the ethanol and air are being quantified per minute. enter your answer to three significant figures in units of ml.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen...
Questions





History, 16.03.2020 19:15

Mathematics, 16.03.2020 19:15





History, 16.03.2020 19:15

Biology, 16.03.2020 19:15




Computers and Technology, 16.03.2020 19:16


Physics, 16.03.2020 19:16


Mathematics, 16.03.2020 19:16

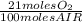 = 0,0406 moles O₂
= 0,0406 moles O₂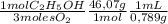 = 0,895 mL of ethanol per minute
= 0,895 mL of ethanol per minute

