
Chemistry, 24.09.2019 20:00 leelee8335
You notice that the water in your friend's swimming pool is cloudy and that the pool walls are discolored at the water line. a quick analysis reveals that the ph of the water is 8.00 when it should be 7.20. the pool is 9.00 m wide, 15.0 m long, and has an average depth of 2.50m. what is the minimum (in the absence of any buffering capacity) volume (ml) of 12.0 wt% h2so4 (sg=1.080) that should be added to return the pool to the desired ph? in ml

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3


Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
You notice that the water in your friend's swimming pool is cloudy and that the pool walls are disco...
Questions

Biology, 12.07.2019 11:30

Mathematics, 12.07.2019 11:30

Business, 12.07.2019 11:30


Mathematics, 12.07.2019 11:30

History, 12.07.2019 11:30

Spanish, 12.07.2019 11:30

Mathematics, 12.07.2019 11:30

English, 12.07.2019 11:30



History, 12.07.2019 11:30





Mathematics, 12.07.2019 11:30



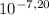 , thus,
, thus, 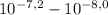 = 5,31x10⁻⁸ M
= 5,31x10⁻⁸ M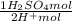 = 8,96x10⁻³ moles of H₂SO₄
= 8,96x10⁻³ moles of H₂SO₄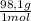 ×
× 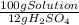 ×
× 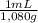 =
= 


