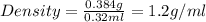Chemistry, 24.09.2019 20:00 prettygirllniyiaa
The active ingredient in aspirin is acetylsalicylic acid. in a lab class, a student uses paper chromatography to isolate another common ingredient of headache remedies. the sample of this ingredient had a mass of 384 mg and a volume of 0.32 cm3. looking at the following data, what was the other ingredient in the headache remedy? white table sugar caffeine sodium chloride d -0.70 g/ml d 1.2 g/ml d 2.2 g/ml

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
The active ingredient in aspirin is acetylsalicylic acid. in a lab class, a student uses paper chrom...
Questions

Mathematics, 02.04.2021 07:00


Mathematics, 02.04.2021 07:00



Physics, 02.04.2021 07:00

Mathematics, 02.04.2021 07:00

Mathematics, 02.04.2021 07:00

Mathematics, 02.04.2021 07:00

Mathematics, 02.04.2021 07:00



Mathematics, 02.04.2021 07:00





Mathematics, 02.04.2021 07:00


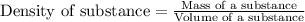
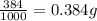 (1g=1000mg)
(1g=1000mg)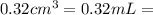 (Conversion factor:
(Conversion factor: 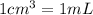 )
)