
Chemistry, 24.09.2019 20:00 AaronMicrosoft15
Amixture of 50 wt% methane, 35 wt% ethane, and 15 wt% propane. determeine the mole fraction of methane.
(can use a basis of 100kg)
b) what is the average molecular weight of the mixture?
for (b) can use m = \sum yi mi where m = average molecular weight, yi= mole fraction of individual substance, mi = molecular weight of individual substance or can also use 1/m = \sum xi / mi where 1/m = average molecular weight, xi = mass fraction of individual substance, mi = molecualr weight of individual substance.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
Amixture of 50 wt% methane, 35 wt% ethane, and 15 wt% propane. determeine the mole fraction of metha...
Questions

Computers and Technology, 22.08.2019 20:30







Mathematics, 22.08.2019 20:40

Biology, 22.08.2019 20:40

Biology, 22.08.2019 20:40


Mathematics, 22.08.2019 20:40

History, 22.08.2019 20:40



Social Studies, 22.08.2019 20:40


Physics, 22.08.2019 20:40


Physics, 22.08.2019 20:40

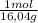 = 31,2 moles
= 31,2 moles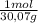 = 11,6 moles
= 11,6 moles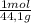 = 3,4 moles
= 3,4 moles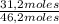 =67,5%
=67,5%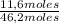 = 25,1%
= 25,1%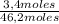 = 7,4%
= 7,4%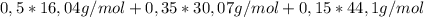 = 25,16g/mol
= 25,16g/mol

