
Chemistry, 24.09.2019 20:30 melidacampos12
Assume that the van der waals b constant for xenon (see table 1c.3- or 1.6 old version - van der waals coefficients) divided by avogadro's number represents the volume of a single xenon atom. assume that xenon atom is spherical, and estimate the radius (in meters) of a xenon atom using the formula vsphere = (4/3)rır3.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

You know the right answer?
Assume that the van der waals b constant for xenon (see table 1c.3- or 1.6 old version - van der waa...
Questions

Mathematics, 20.09.2020 17:01

English, 20.09.2020 17:01





Mathematics, 20.09.2020 17:01

Health, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01


Social Studies, 20.09.2020 17:01

Advanced Placement (AP), 20.09.2020 17:01

Biology, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01





Geography, 20.09.2020 17:01

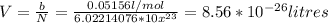
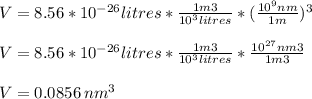
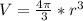
![r=\sqrt[3]{\frac{3V}{4\pi} }= \sqrt[3]{\frac{3*0.0856nm^{3}}{4\pi} }=\sqrt[3]{0.0204 nm^{3} }=0.2734nm](/tpl/images/0259/1334/d0347.png)


