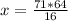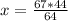The reaction is as follows: ch4 + 202 + co2 + 2h2o if we have 71 kg/hr of ch4 reacting with 67 kg/hr of 02, at what rate co2 will be generated in kg/hr? molecular weight: c-12 kg/kmol h-1 kg/kmol 0 - 16 kg/kmol select one: o a. 46.062 o b. 195.250 o o o c. 1.047 d. 92.125 e. 24.364

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2
You know the right answer?
The reaction is as follows: ch4 + 202 + co2 + 2h2o if we have 71 kg/hr of ch4 reacting with 67 kg/h...
Questions


Computers and Technology, 16.09.2021 01:00

Spanish, 16.09.2021 01:00



Mathematics, 16.09.2021 01:00


Mathematics, 16.09.2021 01:00



Business, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00


Mathematics, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00


Mathematics, 16.09.2021 01:00



History, 16.09.2021 01:00