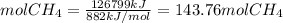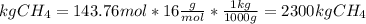The reaction is as follows: ch4 + 202 + co2 + 2h2o the enthalpy of reaction between methane ( 2 ) and oxygen ( 2 ) is exothermic (-882 kj/mol) with respect to the dissipation of methane. it is of interest to generate 126799 kj of energy from the combustion. how many kg of methane will need to be combusted? molecular weight: c-12 kg/kmol h-1 kg/kmol 0 - 16 kg/kmol select one: o a. 2.300 o b. 2300.209 o c. 1.725 o d. 0.009

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
The reaction is as follows: ch4 + 202 + co2 + 2h2o the enthalpy of reaction between methane ( 2 ) a...
Questions

Social Studies, 05.05.2020 16:33




Mathematics, 05.05.2020 16:33






Mathematics, 05.05.2020 16:33


History, 05.05.2020 16:33


Health, 05.05.2020 16:33

Mathematics, 05.05.2020 16:33

History, 05.05.2020 16:33

Biology, 05.05.2020 16:33