
Chemistry, 24.09.2019 22:00 MrTeriffic
Calculate the ph of a water at 25°c that contains 0.6580 mg/l carbonic acid. assume that [h+ ]=[hco3 − ] at equilibrium and neglect the dissociation of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
Calculate the ph of a water at 25°c that contains 0.6580 mg/l carbonic acid. assume that [h+ ]=[hco3...
Questions

Mathematics, 04.11.2021 17:40



Computers and Technology, 04.11.2021 17:40

History, 04.11.2021 17:40

Computers and Technology, 04.11.2021 17:40

Chemistry, 04.11.2021 17:40

English, 04.11.2021 17:40



Mathematics, 04.11.2021 17:40

English, 04.11.2021 17:40

Geography, 04.11.2021 17:40

Computers and Technology, 04.11.2021 17:40






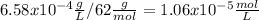
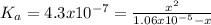
![4.3x10^{-7}.[1.06x10^{-5}-x] = x^{2}\\4.6x10^{-12} - 4.3x10^{-7}x - x^{2} = 0\\x1 = 1.9x10^{-6} \\x2=-2.4x10^{-6}](/tpl/images/0259/3613/28b48.png)
![pH = - Log [H^{+} ] = -Log (1.9x10^{-6}) = 5.7](/tpl/images/0259/3613/91f66.png)



