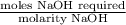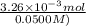Chemistry, 24.09.2019 22:20 PerksInLife
Student weighs out 0.287 g of ascorbic acid (h2ch06), a diprotic acid, into a 250. ml k and dilutes to the mark with distilled water. he plans to titrate the acid with tion olume of naoh ssolution the student will need to add to reach the final equ ur answer to 3 significant digits. ence i don't know submit | privacy oation. all rights reserved. terms of use zo19 micorw

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Student weighs out 0.287 g of ascorbic acid (h2ch06), a diprotic acid, into a 250. ml k and dilutes...
Questions




Social Studies, 10.06.2021 09:10

English, 10.06.2021 09:10



English, 10.06.2021 09:10

Mathematics, 10.06.2021 09:10


Geography, 10.06.2021 09:10

Physics, 10.06.2021 09:10


History, 10.06.2021 09:10




Mathematics, 10.06.2021 09:10

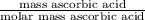
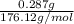
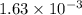 mol
mol
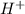 =
= 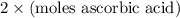
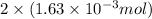
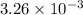 mol
mol