
Chemistry, 24.09.2019 23:11 celibe9391
Aluminum chloride, aich is an inexpensive reagent used in many industrial processes. it is made by treating scrap aluminum with chlorine according to the following equation. 2 aln + 3 cla) → 2 alcl3() a) if 13,49 g of al and 35.45 g of cl2 are allowed to react, how much alcl is produced? b) how many grams of the excess reactant is left? !

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
You know the right answer?
Aluminum chloride, aich is an inexpensive reagent used in many industrial processes. it is made by t...
Questions


Biology, 04.02.2020 23:57






Chemistry, 04.02.2020 23:57

Physics, 04.02.2020 23:57





Business, 04.02.2020 23:57


Health, 04.02.2020 23:57

Geography, 04.02.2020 23:57



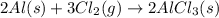
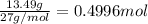
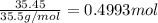
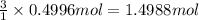 of chlorine gas
of chlorine gas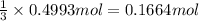 of aluminum
of aluminum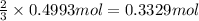 of aluminium chloride .
of aluminium chloride .

