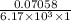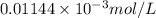Chemistry, 24.09.2019 23:20 bertha4082
The molar absorptivity for aqueous solutions of phenol at 211 nm is 6.17x103l/mol/cm. calculate the linear range of phenol concentrations if the transmittance is to be less than 85% and greater than 7% when the measurements are made in 1cm cell.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
The molar absorptivity for aqueous solutions of phenol at 211 nm is 6.17x103l/mol/cm. calculate the...
Questions

Mathematics, 22.03.2021 19:40


Mathematics, 22.03.2021 19:40

Physics, 22.03.2021 19:40

Physics, 22.03.2021 19:40

Spanish, 22.03.2021 19:40



Chemistry, 22.03.2021 19:40


Mathematics, 22.03.2021 19:40

Mathematics, 22.03.2021 19:40




Biology, 22.03.2021 19:40




Physics, 22.03.2021 19:40

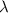 = 211 nm,
= 211 nm, 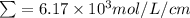
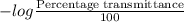
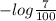
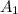 = 1.155
= 1.155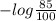
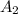 = 0.07058
= 0.07058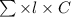
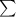 = molar extinction coefficient
= molar extinction coefficient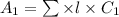
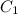 =
= 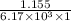
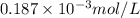
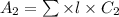
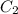 =
=