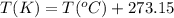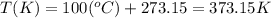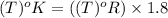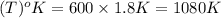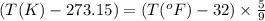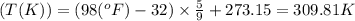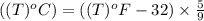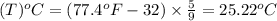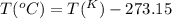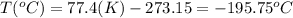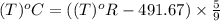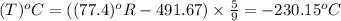Convert the following temperatures to kelvin:
a. 100°c
b. 600°r
c. 98°f
con...

Chemistry, 24.09.2019 23:20 crowzombie9
Convert the following temperatures to kelvin:
a. 100°c
b. 600°r
c. 98°f
convert the following temperatures to °c:
d. 77.4°f
e. 77.4 k
f. 77.4°r

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Questions

Physics, 05.05.2020 10:37

Mathematics, 05.05.2020 10:37





Biology, 05.05.2020 10:37

History, 05.05.2020 10:37

Computers and Technology, 05.05.2020 10:37






Mathematics, 05.05.2020 10:37

Mathematics, 05.05.2020 10:37

Business, 05.05.2020 10:37



Mathematics, 05.05.2020 10:37