
Chemistry, 24.09.2019 23:20 yousifgorgees101
Calculate the atomic radius in cm for the following: a. bcc metal with ao = 0.3226 nm. (enter your answer to three significant figures.) r = cm b. fcc metal with ao = 4.3992 å. (enter your answer to three significant figures.) r = cm

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Calculate the atomic radius in cm for the following: a. bcc metal with ao = 0.3226 nm. (enter your...
Questions

Mathematics, 12.07.2021 16:40

Health, 12.07.2021 16:40


Mathematics, 12.07.2021 16:40



Social Studies, 12.07.2021 16:40

Chemistry, 12.07.2021 16:40

Chemistry, 12.07.2021 16:40

Mathematics, 12.07.2021 16:40





Social Studies, 12.07.2021 16:40


Mathematics, 12.07.2021 16:40

Mathematics, 12.07.2021 16:40

English, 12.07.2021 16:40

English, 12.07.2021 16:40

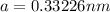
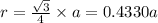
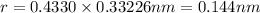
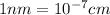
![r=0.144 nm=0.1439\times 10^{-7} cm=1.44\times 10^{-8} cm[\tex]The atomic radius of the metal atom in BCC unit cell is [tex]1.44 \times 10^{-8} cm](/tpl/images/0259/5742/a19f7.png) .
.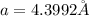
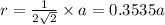
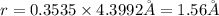
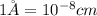
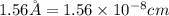
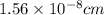 .
.

