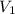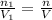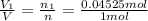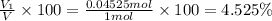Chemistry, 24.09.2019 23:30 zanaplen27
Deep sea divers use a mixture of helium and oxygen to breathe. assume that a diver is going to a depth of 150 feet where the total pressure is 4.42 atm. the partial pressure of oxygen at this depth is to be maintained at 0.20 atm, the same as at sea level. what must be the percent by volume of oxygen in the gas mixture?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Deep sea divers use a mixture of helium and oxygen to breathe. assume that a diver is going to a dep...
Questions

Geography, 26.11.2019 20:31


Physics, 26.11.2019 20:31

English, 26.11.2019 20:31

History, 26.11.2019 20:31

English, 26.11.2019 20:31

Mathematics, 26.11.2019 20:31

Computers and Technology, 26.11.2019 20:31

Biology, 26.11.2019 20:31

Mathematics, 26.11.2019 20:31




Mathematics, 26.11.2019 20:31


Mathematics, 26.11.2019 20:31




History, 26.11.2019 20:31

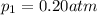
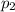
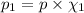 (Dalton law of partial pressure)
(Dalton law of partial pressure)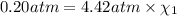
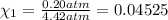
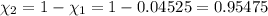
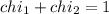
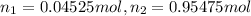
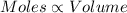 (At temperature and pressure)
(At temperature and pressure)