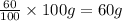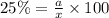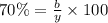Chemistry, 25.09.2019 00:10 19wawrzkeek
Considering an alloy of the two soluble components a and b. determine the masses of the alloy that are in the liquid and solid phases at a given temperature, if the total mass of alloy is 100 g, component b represents 60 % of the alloy, 25 % of the liquid is component b, and 70% of solid is component b.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1


Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Considering an alloy of the two soluble components a and b. determine the masses of the alloy that a...
Questions

Mathematics, 28.05.2021 22:00


Mathematics, 28.05.2021 22:00



History, 28.05.2021 22:10

Spanish, 28.05.2021 22:10

Mathematics, 28.05.2021 22:10

Biology, 28.05.2021 22:10

History, 28.05.2021 22:10

Mathematics, 28.05.2021 22:10



Mathematics, 28.05.2021 22:10


Mathematics, 28.05.2021 22:10


Mathematics, 28.05.2021 22:10


Mathematics, 28.05.2021 22:10